1. Introduction
"I really don't know if this deal is going to close today, or for that matter, ever. Maybe it just shouldn't close at all. It has been a real roller coaster ride up to this point. This morning's fireworks just might have marked the end of this one, Dave."
Dennis Stone, Megacorp's Vice President of Business Development, rushed through lunch with the company's general counsel. It was January 31, 1996. The two young managers had just left a
confrontational and confusing two hour meeting that had been intended to be the closing of Megacorp's acquisition of a small company, Biofirm, Inc. The closing was now delayed as lawyers, shareholders and management representing Biofirm continued to argue among themselves.
Stone, who ran Megacorp’s mergers and acquisitions department, looked back on the past six
months.
"Biofirm has made just about every mistake a small company can make. But I know this business is right for us, and I know management is a good fit. Its great technology. The company fits perfectly into our growth strategy. The price is certainly right, too! We've all invested a lot personally in this one. I don't want it to slip away, just because of unsophisticated shareholders and advisors. Biofirm is an important part of our future."
Megacorp’s general counsel agreed:
"This deal has just been unbelievable. Everywhere we've turned, there's been some kind of problem, surprise or battle. I guess we can only hope that when this is over, its all been worth it."
They headed back to the conference room. They found Dr. Masters, founder and CEO of Biofirm, completing a series of telephone calls to shareholders with the hope of securing a sufficient number of votes to finally close the sale of the business. There was no doubt that Dr. Masters wanted to get the deal done, too. He was Biofirm's largest shareholder. He was exasperated by his fellow shareholders. He was also frustrated by his own lawyer's inability to explain the increasingly complex deal structure, and to find and agree on compromise positions. Masters voiced his thoughts:
"If this deal doesn't close today, I'm resigning. I can't take any more of this. I've given my life to this company. Its obvious that selling to Megacorp is the best opportunity this company will ever see! Why can't the other shareholders see this? If I had a second chance, maybe I'd do some things differently. But how could I have known back then that the decisions I had to make to this business off the ground would come back to haunt me?"
The conference room was quiet and tense. A half million dollar check sat on the conference table,
alongside evidence of three million dollars worth of shares that would accompany the deal.
Cashiers checks for some $200,000 to pay down Biofirm's debts were also delivered by Stone,
including one to the Small Business Administration that would remove a mortgage on the Masters' home to finance reconstruction of Biofirm's manufacturing facility after it was wrecked by Hurricane Hugo. Stacks of paper awaited signature, representing hundreds of hours of work by numerous professionals.
2. Megacorp
Megacorp was founded fifty years ago by a young veterinary school graduate, Dr. Jones, to breed laboratory animals. Megacorp operated 25 production facilities, some of which were overseas. The Company produced biological or "living" products for use in biomedical research, consumer product safety testing,, and animal health care. Megacorp also produced biological raw materials for human therapeutic applications.
Megacorp's mission statement, to be "the premiere company contributing to the search for healthier lives" reflected a clear growth orientation compared to the company's historical mission of just being "the world leader in laboratory animals."
The company's "core" business continued to be the production of genetically defined and specific
pathogen free (or "clean") rats and mice for laboratory research. Megacorp grew rapidly in the
1960's and 1970's through acquisitions in its core business. During the 1980's, the company
diversified into new product lines in the biotechnology and biomedical fields, including production of monoclonal antibodies for therapeutic purposes, animal organs for human transplantation, and embryo cryopreservation for human and animal fertility applications. Despite this incremental broadening of the product portfolio, the company derived in excess of 80% of its revenues from the sale of laboratory animals.
At the same time, Megacorp's core business had been declining in unit volume in the range of 1-2% per year for the last several years. The industry had undertaken systematic efforts to reduce unnecessary or duplicative animal tests during that time. Mergers in the pharmaceutical industry had consolidated R&D labs. Increased pressure on pharmaceutical companies, particularly from the Clinton Administration, led to price sensitivity for the first time in fifty years. As pharmaceutical companies were being pressured to limit their drug price increases, they encouraged suppliers such as Megacorp to limit their own price increases. New substitute techniques to using laboratory animals had also emerged such adjunctive in vitro technologies. As a result, the developed market for the core business was thought to be $250 million in 1994. Megacorp was already the market leader throughout the world with dominant market share in the US and Europe and rapidly increasing share in Japan.
Megacorp had been a financial success throughout its fifty-year history. It had consistently maintained operating margins at or near 20% and had grown in both sales and earnings on an historical average basis of 15-20% per year. Megacorp achieved P/E ratios ranging from a low of 20 to a high of 60 during the 1980's. Entering the 1990's, Megacorp found it increasingly difficult to achieve 15-20% growth in earnings in the laboratory animal businesses. By early 1993, the company was busy diversifying, into new but closely related areas.
Acquisitions were sought in businesses that provided either:
a) process technology leverage, being able to build upon Megacorp's core competency of large-scale production of contaminant-free biologicals or
b) market leverage, being able to exploit Megacorp's strong customer relationships within the biomedical research community.
Stone served as the new ventures manager. Good management, strong technology, and growth markets were the key criteria that he sought in its acquisition candidates. Megacorp's new ventures process had several basic steps. After identifying a potential acquisition, a preliminary analysis of the company and its market was done. Particular emphasis was placed on the "strategic fit" of the candidate, the possible "synergies", and the current and potential financial retums. A discounted cash flow valuation of the business followed, setting the parameters for price negotiations. A non-binding letter of intent setting forth the proposed deal structure and price would then be negotiated. This letter of intent would be brought to the Board of Directors for approval. Once approved, an extensive due diligence investigation of the company would be conducted, including financial, legal and technology "audits". If the due diligence was successful, detailed legal agreements were negotiated, and the deal would then be brought to the Board for final approval.
Stone had recently completed an acquisition that had been a "hit" for Megacorp, and naturally, he wanted to complete more like it. In 1992, he had acquired a small company called CleanEgg, which produced specific pathogen free eggs for use in vaccine production and research. CleanEgg's egg technology was closely related to Megacorp's core competency of producing "clean" living, or biological, products. The distribution channel was largely the same (biomedical researchers and biopharmaceutical companies). Management was solid. CleanEgg was also number one in the its clean egg market. In the year since the acquisition, CleanEgg had performed exceptionally well, growing, rapidly and improving its market position. Stone generalized from this experience:
"In order to be a successful new ventures company, we need to add a CleanEgg like company to our portfolio every 12 months. That means sorting through hundreds of possibilities and being smart about how we set our priorities and pursue a select few opportunities. We don't need a big breakthrough deal to be successful, just a steady flow of small winners."
Stone's strategy was to participate in emerging technologies that might replace Megacorp's historic lab animal technology model, or in his own words,
"What new technologies might put us and our traditional lab animal testing technology out of business? How can get we involved learning and using these technologies? I know that most large companies have great difficulty doing this. However, a firm has to obsolete its own products and technologies in order to survive."
He preferred acquisitions rather than strategic alliances, technology licensing or internal new product development. Acquisitions were "cleaner", providing Megacorp with control and less risk of future litigation. Megacorp’s own internal R&D had been so focused on lab animal technology that its scientists had shown little success in developing new products outside "the core" business.
One emerging industry that intrigued Stone involved the use of alternative materials or methods to conduct the tests required by biomedical researchers. Within this area, Stone and his colleagues were particularly interested in the in-vitro testing business. One in-vitro technique is known as the MLA assay, a specialized reagent used to test for the presence of certain diseases. The MLA had already been recognized by US regulatory authorities as a fully acceptable in-vitro alternative to the use of a whole animal.
3 The MLA Industry
3.1 The Technology
Dr. Masters, CEO of Biofirm, had been one of the founders of the MLA industry. MLA was based on endotoxin. Endotoxin is a component of the cell wall of gram negative bacteria. It is found almost everywhere in nature, especially in water. Also known as "pyrogen" for its fever
producing effect, endotoxin is generally harmless to humans except when it enters the blood
stream. At levels as small as a few hundred nanograms, endotoxins can cause fever, shock, hemorrhage, and ultimately death. For this reason, medicines, fluids, and medical equipment which are used in intravenous therapy must be free of endotoxin contamination.
The rabbit fever test developed in the 1940's was the standard test for endotoxins, until the approval of the MLA test by the FDA in the 1980's. In the rabbit test, healthy rabbits were injected intravenously with a sample of rug- or with a wash from a medical device. The temperature of the rabbits was monitored for three hours. If a one degree or greater rise in temperature was observed, the product was considered pyrogenic. As many as one million rabbit tests were performed annually prior to US regulatory acceptance of the MLA alternative.
In the 1960's, a university-based pathologist observed a large number of horseshoe crabs dying on a beach on the northeast coast of the US. He found that their blood was clotted and that they were infected by gram negative bacteria. A hematologist colleague of the pathologist also studied the crab's disease because it mimicked the course of human infection. The hematologist's research showed that the blood cells of the horseshoe crab were responsible for the clotting, and that endotoxin from the gram negative bacteria was the cause. From these blood cells, the hematologist developed a test reagent and began research on testing for endotoxins in patients with serious infections. He named this reagent MLA.
In 1969, Dr. Masters, then a university researcher, was searching for a method to conduct pyrogen testing in radioactive tracers used in diagnostic nuclear medicine procedures. Because of the short life and biohazard potential, radioactive tracers could not be tested by injecting rabbits. Dr. Masters was aware of the new work with the MLA reagent. Masters tried the reagent and found that it worked in nuclear medicine applications and published his findings. His did more research to show that endotoxin caused aseptic meningitis and that the MLA test could detect the endotoxin. Masters then did studies with colleagues in the FDA that showed MLA to be an extremely useful tool in evaluating drugs and devices which had caused patient reactions.
As the merits of the MLA test were identified and expanded, the FDA and the pharmaceutical industry began to develop standards and regulations to allow widespread use. The drug companies began providing multi-million dollar orders for suppliers in the early 1980's. In December 1987, the FDA issued guidelines outlining the steps necessary to obtain approval for MLA to be used in lieu of the rabbit fever test for release testing of drugs and medical devices.
3.2 The Market
The estimated market for the MLA reagent and related test materials to detect endotoxins was $50 million in 1994. The end-users of MLA tests were comprised principally of drug manufacturers, biotech companies and medical device manufacturers. In excess of 90% of all MLA applications were for "fever" or pyrogenecity testing of FDA approved, injectable human drugs. The US market was estimated to comprise 60% of the total market for MLA products, or $30 million. The European and Japanese markets were estimated to be 40% of the total, or $20 million. The highest rate of growth in the near-term was expected in Western Europe, where regulatory acceptance of the MLA test for final product safety testing- had occurred in several European counties in 1993. By the year 2000, the US market was expected to be half of the world market, with Europe and Japan taking the majority of the balance. Industry observers expected the MLA business to grow at 15% a year, the rate realized since the FDA approval of the MLA test in December 1987. The industry was highly profitable, with the three leading MLA manufacturers maintaining estimated operating margins of 30%. The market was sensitive to quality, service and price, in descending order of importance.
New markets were expected to open up in the next five to ten years for MLA testing in developing economies in Eastern Europe and Asia. In the established markets, new applications for the technology were expected as well. The biotech industry was expected to use more MLA testing, as nearly all existing and anticipated biotech products were administered by injection. The veterinary MLA testing industry was also expected to experience substantial -growth as veterinary pharmaceutical companies increased their MLA testing activity in response to new regulations. In addition, regulatory changes impacting manufacturing process quality testing were expected to increase the level of raw materials testing. The opportunity in new markets and for new applications equaled or exceeded the current market for existing applications. This brought the total long term opportunity in the MLA market in excess of $100 million in annual revenues.
3.3 The Suppliers
There were four companies in the MLA industry, one of which was Biofirm. Stone's due diligence revealed information about Biofirm’s competitors.
o Competitor A: a privately held firm founded in 1977. This company was the historical market leader with 1993 sales estimated at $13 million. This represented a 45% share of the commercially-supplied market (i.e., excluding in-house production by biotech and pharmaceutical companies). The company had lost market share to its competition in the last few years due to product quality problems and service deficiencies. It nevertheless continued to enjoy a high level of brand recognition throughout the world. The firm had not significantly increased the price of its products since 1985 in an attempt to avoid further market share deterioration.
o Competitor B: a division of a publicly-held company. The company’s MLA operation had grown rapidly in recent years, principally at the expense of Competitor A with MLA revenues standing at about $12 million in annual sales, a 40% market share. The company had used extensive marketing and a large direct sales force to grow in the US. Its products were known for good overall quality in the high-end se-segment and held a significant price premium.
o Competitor C: a privately-held firm. With less than $1 million in sales despite a long- operating history, this company competed primarily on price and provided only limited technical service.
Twenty companies had tried to enter the MLA industry since 1980 without success. The technical "know-how", the specificity of customer needs, and the horrors-ors of the FDA approval process had proven strong barriers to entry.
In June 1993, Megacorp's international management team met for the annual corporate meeting, During an "all-hands" session, Stone reviewed with the management group the corporate development priorities. After lively discussion, the group determined that pursuit of an MLA acquisition should be a priority. Starting an MLA business from Megacorp's internal R&D was
deemed impractical given the highly specialized nature of the process and the long lead-time required to acquire FDA licenses to operate the business.
Stone visited Biofirm's largest competitor, Competitor A. This was not the first time such a visit had been made. Competitor A had flatly refused several overtures from Megacorp over the past decade for a buy-out. Its CEO and sole owner welcomed the Megacorp group and gave a plant tour. After a friendly and open discussion, he indicated that he was beginning to think about selling the business. The CEO was past retirement age and indicated that he was in ill health. When pressed as to the terms of a possible sale, the CEO offered that he would consider selling- the business for "$40 million dollars or so", a price that was a "bargain" for this business. Stone figured to himself, "$40 million on sales of $13 million with earnings at perhaps 30% ... no way. He really doesn't want to sell".
However, during the plant tour, an employee mentioned in passing, that the MLA inventory was building significantly. When Stone asked why, he said that the firm was losing market share to a new competitor that had entered the market. The CEO later identified that competitor, then unknown to Megacorp, as Biofirm.
As Stone left, he said to Megacorp's CEO:
"Since getting into the MLA business is a strategic priority, maybe we should finally take a look at the competition. The CEO's price is awfully high, and I doubt he will ever actually sell the business. We can't pay that kind of price ... the multiples would be outrageous. Competitor B sounds like an interesting acquisition candidate. I know it's a diversified public biotech company, but they may want to sell just the MLA product line. And maybe I'll track down this Biofirm. I've never heard anyone in the MLA business mention them. They're probably too small to bother with but at least a visit will help us build our knowledge base about this industry."
Megacorp's CEO agreed. He encouraged Stone to take a careful look at the competitors:
"I agree with you that the old fellow will never sell the business. And the price is outrageous. We need critical mass in this business in order to be successful. I doubt Biofirm can offer us that. Focus on Competitor B, but check into Biofirm, too. Where do you think Biofirm is located, anyway? Its odd that we've never even heard of them. Our people have followed the MLA industry for years, and no one has ever mentioned to me that a new player entered the market."
4. BIOFIRM
It took several hours of research and a couple of days of calls to finally track down Biofirm. Stone reached Dr. Masters by telephone, expressed an interest in his business, and congratulated him on taking on his competitors successful . Stone suggested a visit to Biofirm sometime in the next couple of weeks, to "discuss areas where we might be able to work together." Masters was very receptive. "Do you think Megacorp would ever consider buying us?" Stone,
surprised but pleased by Masters' frankness, responded:
"Well, Doctor, I think that's a real possibility. Lets discuss it when I visit. I have to warn you, though, that we're intent on getting into the business in the biggest way possible. Our strategy is to be number one in every market we play in. We'll be looking at all the players, and we can't let ourselves get locked into any one option until we've evaluated all of the viable ones."
Masters laughed in response:
"Competitor A will never sell, Mr. Stone. Competitor B won't sell either. I know them both well. Both companies are happy as they are and enjoy the profitability of the MLA product line."
Biofirm had been founded in 1987 by Dr. Masters. It’s mission was "to offer the most
interference resistant, reliable, and stable pyrogen test for pharmaceutical MLA testing."
Biofirm's production facility and gel clot product were first approved by the FDA in 1989. Biofirm doubled its sales in each of the three years following FDA approval. For 1993, the Company earned $800,000 before-tax on $2 million in sales, an impressive 40% net margin. More than two-thirds of its existing business was the result of conversions of customers from one of the two market leaders.
The innovation in MLA technology developed by Biofirm was its proprietary method of chemically formulating the gel clot reagent to a variety of sensitivities as determined by its customers' specific requirements. This allowed the reagent to achieve compatibility across a broad range of tested products, along with a consistent and unequivocal low end point at all levels of sensitivity. Master’s held several patents on his chemical formulation process. Interference with test results from the product being tested was shown to be minimal. This resistance to interference was a key competitive advantage. The Biofirm reagent had also gained recognition for its performance in terms of reliability, ease of use, extended bench stability, clot firmness (leading to easier, more objective interpretation), and, conformance to label claims for shelf life.
Biofirm was a classic technology startup in terms of financing. Masters first used nearly all of his family's savings. He later turned to neighbors and professional acquaintances for additional money. Because the MLA was regulated by the FDA as a "drug", Biofirm had required nearly three years of start-up time to acquire the necessary FDA licenses and permits. During this period, the Company had no cash flow and was unable to obtain a bank loan. There was no interest on the part of the venture capital community, as the market opportunity then seemed rather limited. Besides, Masters had never run a business before.
Biofirm’s shareholder group eventually grew to sixteen persons by 1993. All of these investors
were local small business owners. The total amount of capital raised from them was $300,000, with each investor paying $10 per share for the total of 3,000 shares issued. The typical shareholder of Biofirm invested $10,000 to $15,000 in the company. Their expectation was that
they would receive their investment back as soon as cash flow permitted and would maintain their
equity position for any "upside" payout when the company went public or was sold. Masters and his chief operating officer together owned less than a majority of the company’s stock.
Masters had been careful to keep his payroll and expenses as low as possible during the start-up phase. He had periodically enlisted the help of colleagues and other professionals to solve operational problems and had offered them a "piece of the company" if the business was successful in lieu of cash payment.
Over the next several weeks, Stone and Masters continued their telephone conversations, getting to know each other and their respective businesses. Stone provided Masters with a confidentiality agreement, which protected both sides from misuse of the other's confidential information. This agreement provided that Megacorp was not prevented from pursuing other MLA companies while engaged in confidential discussions with Biofirm. Masters provided Stone with extensive written information on the technology, the industry, the competition and his company. Stone thought to himself:
"You just never know where the next deal is going to come from. I never even heard of Biofirm before last week. It seems too good to be true... he wants very much to sell and we want to buy. I'll bet his financials are lousy. Why else would he be so anxious to sell? His openness about his business and his personal goals is refreshing. But, why hasn't he sold out already? Why would he seem to be just waiting for my call?"
Within a week, Stone and Megacorp's Chief Scientific Officer visited Biofirm. The group reviewed Biofirm's history, technology, strategy, key personnel, financial performance, customer list, and numerous other areas of the business. While Biofirm was quite small, it enjoyed the operating margins that the MLA business was known for, namely 30%.
Stone remained enthusiastic and began to see Biofirm as a real alternative to Competitor A.
Unfortunately, Megacorp's CEO needed more convincing:
"Biofirm is awfully small. And they're a long way from being number one in their market. Masters seems very strong technically, but he doesn't appear to be a manager. If Competitor A ever gets their act together they might crush Biofirm. I don't want to give up on them yet. And Competitor B is a real alternative for us."
On the other hand, Megacorp’s Chief Scientific Officer believed that Biofirm had great technology and could grow rapidly with Megacorp’s manufacturing and distribution strength behind it. Stone agreed:
"So what if they're small, they've got the best technology. We can grow them into number one in two or three years if we're really committed. We've got the marketing and salesforce that they lack. I like Masters. Let's take this one as far as we can. Our CEO can be convinced if we pull this together and make it an attractive opportunity."
5. The Dance Begins ...
Stone phoned Masters the next day. He proposed to Masters a management earn-out deal:
"Dr. Masters, we like senior management of newly acquired companies to have an incentive to really grow the top and bottom lines. We want you to be investing all of your time and energy into taking Biofirm to the next level. We think you and your team should be rewarded for a real breakthrough level of growth. For this reason, we'd like to discuss with you an earn-out on this deal. I know we haven't put together a deal structure of price yet, but the earn-out needs to be agreed to up-front. Do you have any thoughts on this?"
Masters was surprised by Stone's query. He was familiar with the concept of a management
bonus in this kind of deal, but was uncertain about how an earn-out would be structured. He
owned 28% of the equity in Biofirm. It should be worth something right up front. Now, Stone
was talking about future incentives.
Stone described a specific earn-out formula, explaining that it had worked before for other entrepreneurs in similar situations. Masters would receive 10% of the incremental earnings Biofirm achieved annually above 50% year-over-year growth in sales. A 5% earn-out would also be provided to Masters’ chief operating officer. The earn-out would then be included in employment agreements with a three-year term and one year's severance in the event of a termination for reasons other than "cause". The earn-out could result in significant bonuses for Biofirm's management. Stone explained further:
"The earn-out is triggered only at a "knock your socks off' growth target. We won't have to include the earn-out in the valuation of the business, since we won't be assuming growth rates that high. It's basically all upside for both us, a real win-win. And, I think it will motivate your management team to drive the business to really grow. Besides, it is you that has really created the value in this company. Your deserve to get a "kicker" if you keep on performing.
Over the next four weeks, Stone flew from Boston to meet with Masters several times. Stone also visited Competitor B’s CEO who politely but unequivocally declined to discuss an acquisition:
"I love the MLA business. It generates great profits and supports some other activities we're pursuing. The market is fairly small but it's stable. And I'm beating Competitor A in the marketplace. I need to keep an eye on a small new player. I think its called Biofirm. It seems to have good technology and service."
Stone prepared a written analysis of the MLA industry and the Biofirm opportunity. This analysis would become the basis upon which Megacorp's Board of Directors would be introduced to MLA and Biofirm. It contained a detailed analysis of the MLA market, based on interviews with the leading MLA users in the US and selected companies in Europe and Japan. It also proposed a strategy for Megacorp that used Biofirm's technology to launch an attack on Competitor A and Competitor B around the world. Extensive financial analysis was also included. All of Bighorn’s competitors players were privately-held, or not "material" to their publicly-held parent companies, thereby limiting required disclosure under SEC rules. The industry was too small for coverage by Wall Street analysts. Despite hundreds of scientific publications on MLA, there was no published data on the business of MLA and no recognized independent industry expert. Stone had to build his analysis from direct conversations with Biofirm competitors and various customers (pharmaceutical and biotech companies).
Stone called a meeting of the senior executive team at Megacorp and presented his MLA analysis.
An executive responded for the group:
"Well, I'm still concerned that Biofirm is too small. But at this point, I don't see any other viable way for us to get into this market. Lets do a valuation. Why don't you see if you can get a price out of Masters. Maybe we won't be able to get into the same ballpark with him. Don't make any commitments, though. I'm not sure our Board will want us to take on something so small. In the past they have been critical when we focused on small opportunities with limited potential."
6. Valuing the Company
Stone now had to come up with a valuation for Biofirm and chose the best deal structure. These
would then be negotiated with Biofirm shareholders.
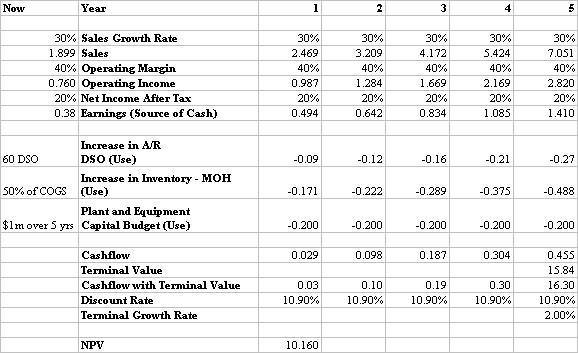
Stone had seen other companies use valuation techniques based on multiples of sales, multiples of earnings, book value, or a combination of all three. However, he preferred a discounted cash flow approach. He ran Megacorp's discounted cash flow (DCF) computer spreadsheet model using Biofirm's historical unaudited financial statements. Appendix 2 shows a boilerplate spreadsheet for the analysis.
The DCF model requires certain assumption be made regarding Biofirm’s:
o sales growth,
o profitability,
o capital requirements, for things such as manufacturing, capacity,
o the discount rate for the cost of capital
o and terminal growth rate, which is the growth rate applied to sales after the initial five year valuation period for asset valuation
Stone felt that Biofirm could increase sales at a rate of 30% over the next five years, and that its profitability would continue in the 30% pre-tax net income range. These kind of growth rates, not seen at Megacorp since the late 1970's, combined with an extraordinary level of profitability would clearly drive the valuation well in excess of $10 million assuming he used an industry standard weighted cost of capital of 10%.
Stone considered the situation further because he thought $10 million to be too high, both for
external market and internal management reasons.
"We need to be very careful here, since our valuation in the acquisition will surely end up as our business plan for the following year once we run the business. That's the Board's way of insuring that we believe in the valuation, tying our bonuses to achieving the assumptions in the valuation.
If we can justify paying a reasonable multiple of sales and earnings for this business, we don't need a high valuation. I'll bet just a 10% or 15% growth rate will cover a decent multiple on this deal, since profitability is so high. Less than 10 times EBIT and 2 times sales would be attractive..."
Stone discussed his valuation thoughts with Megacorp's Chief Financial Officer and revealed his
target:
"We need to look at earnings multiples. There just aren't very many businesses that generate 40% pre-tax profit. With sales of $1.889 million 1993, Biofirm had earnings before income tax of $750,000. Ten times EBIT would be $7.5 million. If we could get the business for half that, or $4 million, we'll be in great shape. Then, the sales multiple would be 2X, and the earnings multiple only 5X. The internal rate of return will easily beat our 15% hurdle rate, based on this price. I need to get the discounted cashflow valuation to support a $4 million price, and I can't imagine that it won't, given the fundamentals of the business."
The Chief Financial Officer reminded Stone of his position on valuation of new businesses:
"Dennis, you know that the valuation will be the operating plan for this one. I don't want to commit to sales growth of more than 10%, at least initially. We don't know a thing about the MLA industry, and this company is a distant third in the market. And remember that their pre-tax net income won't hold at 40%, since we'll be allocating overhead costs from our operation, and direct sales and marketing costs, too. And don't forget the capital requirements. We always underestimate the capital required to grow a small business. Let me know how the value comes out. I suspect that the discount rate assumption we now use needs to be increased to reflect the risk in this deal."
Stone went to work on the his valuation, following the process outlined in Appendix 1, and using the financial data and template provided in Appendix 2. He had to make the following decisions:
o Growth rate: Should he follow the CFO’s advise, Stone would use a conservative 10% growth in sales as opposed to the 30% that he himself believed was highly possible in the next three years. The lower number would drive down the valuation.
o 0perating Margins: Biofirm's gross margin was running at about 40% of sales.. Should he use this, or drop it by 10% or so to reflect a business with increasing expenses in production and selling? Stone thought the latter more prudent.
o Profitability: Biofirm's pre-tax net income was currently running at a 25%. Once again, Stone thought that this might be reduced to a more conservative 15% to 20%.
o Capital Requirement: Stone studied Master’s growth plans and estimated that about a $1 million would be needed to expand production capacity as the business grew over the next several years.
o Discount Rate for Cost of Capital: A higher discount rate reflects greater risk, and drives down the valuation. Stone used his company's economic value-added weighted cost of capital: 10.9%. This was a standard discount rate used for cashflow valuations of new ventures.
o The Terminal Growth Rate: Stone thought that this should be set at 4%. (Dennis, we need to explain this further with a sentence or two.)
There was also the matter of goodwill. Stone spoke with his CFO further:
"There's going to be a big goodwill hit on this deal. The real value in Biofirm is its FDA licenses, and there's no value attributed to them on the balance sheet. We're going to be looking at maybe a couple of million dollars of goodwill, since the net book value is only around a million dollars."
In the valuation of the business, this goodwill would have to amortized and subtracted from the operating profits in the DCF model. A conservative amortization period was deemed to be 20 years.
"We'll be looking at a $ 100,000 hit to earnings annually, of a small base, with very
minor tax benefit".
Finally, there was the issue of Megacorp Board approval. Any offer made to purchase Biofirm
would have to represent real value.
"Our Board should recognize the conservatism of the assumptions used in the
valuation. Hopefully they'll see that the real value of this business is perhaps twice what we will end up spending, buy it."
Based on his prior experience, Stone also know that any offer of more than $5 million would invoke elaborate deliberations by Megacorp's Board. While this was by no means a show-stopper for an acquisition, it would make things more complicated. The Board would also approve of the management earn-out plan that Stone had proposed. Biofirm would be nothing without Masters and his number two man, at least for two or three years. However, the earn-out would not be reflected in the valuation because it would only be triggered after 50% growth. Wanting to play it
very conservative, Stone was going to use a 10% sales growth in his calculations.
Stone also had to consider how to best structure the deal. There were four basic options:
Option 1: buying shares with cash (a cash merger);
Option 2: buying shares with Megacorp shares (a stock merger);
Option 3: buying assets with cash;
Option 4: buying assets with Megacorp shares
Stone made a list of the benefits and drawbacks of each option, as shown in Appendix 3.
Megacorp could gain control of Biofirm by acquiring 50.1% of the equity (Options 1 or 2). The problem with this approach would be that it would not allow full consolidation of Biofirm's financial results into the Megacorp financial statements (Dennis, so who cares?) . Similarly, a joint venture or other form of partnership would not offer Megacorp the benefits associated with full ownership and control of the business.
Stone's liked Option 3, buying assets with cash. This structure would be the simplest deal to complete, and most importantly would offer the greatest protection to Megacorp. The asset purchase structure would allow Megacorp to buy only what it really wanted while avoiding assuming unwanted liabilities. Biofirm was a small, relatively new company whose founder was a scientist, not a businessman. There could be serious operational, distribution, or patent problems giving cause for litigation. By acquiring only Biofirm's assets, as opposed to the legal entity, Megacorp would avoid any unknown or contingent liability, Any type of merger (Options 1 and 2) would be just the opposite as a matter of law.
Was Stone being too cautious?
"Small businesses are famous for having unknown liabilities, like patent infringement, so buying assets is the only way to go in this type of situation. However, the Biofirm seems totally clean from a liability standpoint."
Using Megacorp's shares to buy assets (Option 4 above) also had its problems.
"Share deals are always difficult to manage. They add a level of complexity, when that's the last thing you need in a small deal. Besides, Megacorp hates doing share deals since it dilutes existing shareholders to issue new shares to buy a company. Plus, you've got the SEC registration process, which means delay, expense and more lawyers. I know, I am one of them!"
There was also the matter of taxes. Option 4, swapping shares for assets, if structured in accordance with detailed IRS regulations would probably qualify as a tax reorganization. This
meant lots of paperwork. Option 2, a stock merger, would normally offer Biofirm shareholders a way to avoid paying capital gains taxes. However, do to its prior history of mergers and complex IRS regulations, Megacorp's treasury shares were "tainted". As a result, Stone did not believe that Masters and his investors would receive a tax-free capital appreciation.
Appendix 1
Discounted Cash Flow Valuation Process: 10 Simple Steps
1. Establish Base Point (from current P&L) for Sales, Gross Margins, Net Margins -- so as to get Operating Income and Net Income before tax.
2 Determine market growth rate, establish projections for 5 years on Sales.
3. Project operating income on reasonable margins and net income.
4. Look at both Receivables and Materials/Payables to see if cash is being consumed to the extent that net income needs to be adjusted. (Once you buy the business, you can generate more cash by improving the collection of receivables or the reduction of inventories.) The balance sheet might also have to be cleaned up in terms of retiring undesirable debt.
5. Look to Business Plan and determine reasonable capital investments into the business for plant and equipment, and other types of expansion.
6. Determine tax rate.
7. Determine Discount Rate -- The companies estimated weighted cost of capital, adjusted for extraordinary risk on particular deals, is a commonly used discount rate.
8. Layout Cashflow over five years, then discount back into today's dollars.
9. Establish terminal value. A simple, useful way is to take net income for year five, and divide it by your discount rate, adjusted for the terminal growth rate. Then, discount that back to today's dollars.
10. Add 8 and 9 to get your valuation.
Appendix 2
Financial Data Summary Form

Capital Investments = $1 million spread out over five years.
Terminal Value = (Year 5 Net Income) / (Project Discount Rate - Terminal Growth Rate)
For students:
What happens to the valuation if the sales growth rate is lowered, or raised, by 10% a year ?
Or, the Gross Margin is lowered to 50%, and Operating Margin to 30% ?
Or, conversely, the Gross Margin is raised to 70% and the Operating Margin raised to 50% ?
Likewise, what if this deal was being done in Brazil ? How does the discount rate drive the valuation ?
Note further that Megacorp liked to make these valuation spreadsheets the actual operating plan for the acquired company -- the plan to beat for management earnouts in the order of 10 cents on every dollar of after tax earnings above plan. This was also a way of providing extra value to management without having to pay dollars to all of the seller’s shareholders up front.
Appendix 3
Deal Structure Guide
For Buyer For Seller
Type of Deal
|
||||
Cash for Shares
|
No SEC registration
No consents, assignments of intellectual property / assets |
Liability assumption
Long form documents |
No double taxation (corporate /dividend income)
No residual liability
Cash in hand |
No tax deferral on capital gain |
Shares for Shares
|
Maintain cash | SEC registration
Long form documents
|
Depends of performance of parent company’s stock | If company not publicly traded, illiquid. |
Cash for Assets
|
Liability insulation
No SEC registration
Tax deduction for goodwill amortization
Low deal expenses
|
Consents, assignments |
Short form documents
Cash in hand |
Double tax on C Corporations
Potential residual liabilities
No individual tax deferral |
| Shares for Assets | Liability insulation
Short form documents |
SEC registration
No tax deduction for goodwill amortization
High deal expenses
Need Board of Directors signatures |
No double taxation
Tax deferral |
Potential residual liabilities
50% withholding requirement
Potential IRS rejection of reorganization |